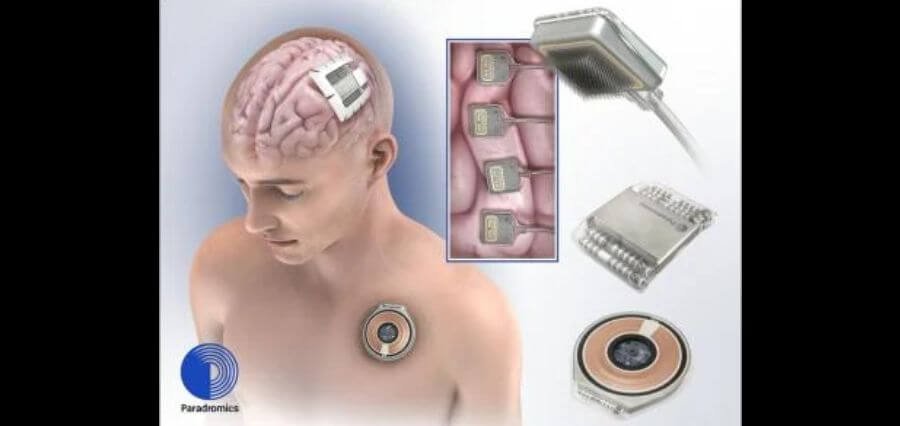
Paradromics, a brain implant company made some announcements regarding the first in-human trial which is to be conducted next year and the company also reiterated that it is taking the necessary steps to launch its official patient registry. Paradromics is on a mission to build a brain computer interface or BCI. The goal of BCI can be used to decipher brain signals and transmit them to external technologies.
The Paradromics system is initially designed to serve as an assistive communication device that can turn brain signals into outputs like text or synthesized speech. It can be used by patients with paralysis to regain their ability to communicate effectively. Research has been going in academia since decades and Elon Musk’s Neuralink have been developing their own systems. Prominent industry leaders like Bill Gates and Jeff Bezos have also made investments in a company named Synchron.
Paradromics BCI is designed to be inserted directly into the brain tissue, which indicates that the patient must undergo a major surgery. The company still has to clear major hurdles with US Food Drug and Administration before the technology becomes available in the public domain. The company aims to carry out its first human trail in 2025.
FDA has accepted Paradromics proposal for its Total Product Lifecycle Advisory Program or TAP on Monday. TAP is designed to be used as a communication bridge between the FDA and the companies that have already received the agency’s Breakthrough Device Designation. A press release stated that Paradromics has received the recognition twice from the agency.
Sometimes, the response time of FDA is slower as it is working with thousands of organizations at a time. As a result of access to open line communication the company and the agency will be on the same page. CEO of Paradromics Matt Angel said “We appreciate the access to the TAP program and want to deliver the best possible device in the safest possible timeline.”
Read More: Click Here